On December 31, 2021, Shanghai Allist Pharmaceutical Technology Co., Ltd. (hereinafter referred to as "Allist", stock symbol: 688578) announced that its Class 1 innovative drug Furmonertinib Mesilate (hereinafter referred to as "Furmonertinib"), developed in-house, was listed in the "Guidelines for Clinical Application of Novel Antitumor Drugs (2021)" issued by the National Health Commission of the People's Republic of China.
About the "Guidelines for Clinical Application of Novel Antitumor Drugs"
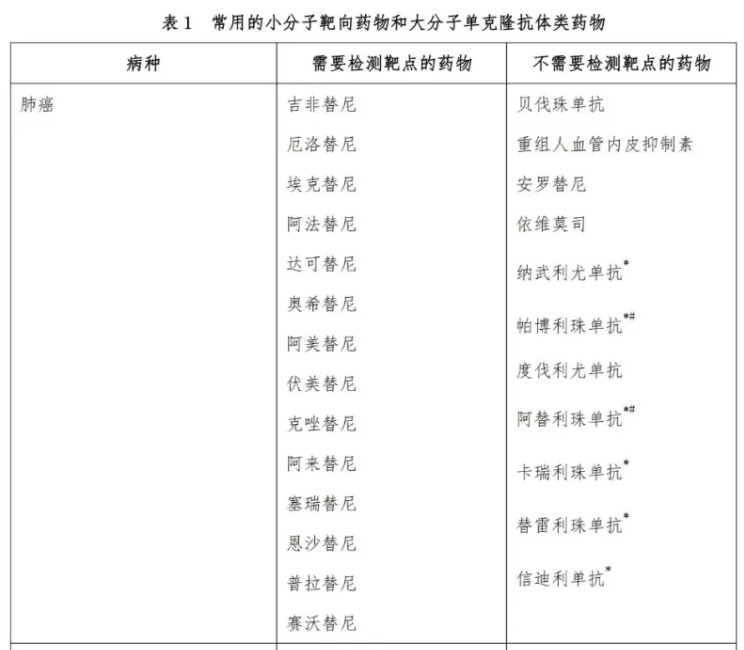
To further standardize the rational clinical application of antitumor drugs, the National Health Commission organized the Committee of Experts on Rational Drug Use to jointly prepare the "Guidelines for Clinical Application of Novel Antitumor Drugs" (hereinafter referred to as "Guidelines 2021"). Since initial release in 2018, the Guidelines have been updated once a year and have become the "benchmark document" for the standardized use of new anti-cancer drugs across various fields in China.
On December 27, 2021, the National Health Commission released Guidelines 2021. Compared with the 2020 edition, the new version saw the addition of 5 more types of cancer and 29 new indications for new antitumor drugs.